Mose et al. (Jørgensen lab) [Nature Chemistry]
There are whole bunch of messy electrocyclic reactions in the paper and nice projections explaining diastereoselectivity. They made me nostalgic about reading OC texbooks.
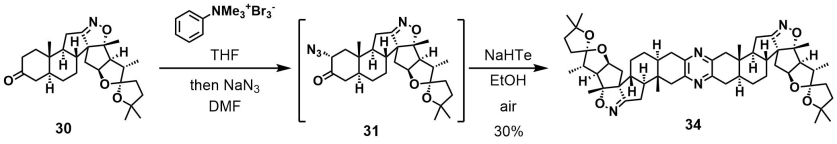
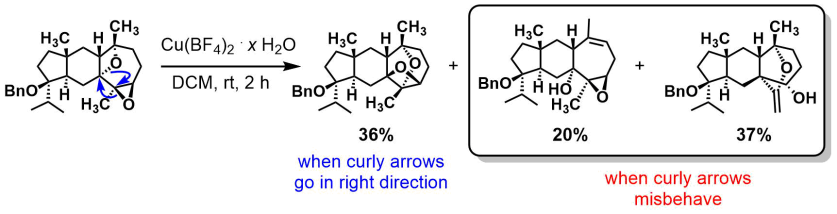
Hugelshofer and Magauer [JACS]
Want something even cooler, here’s a dyotropic reaction! Characterizing the side-products must have been a lot of fun. As well as monitoring the progress (the major undesired product had the same Rf as the starting material, and good luck with crude NMR). Heads up from amphoteros.
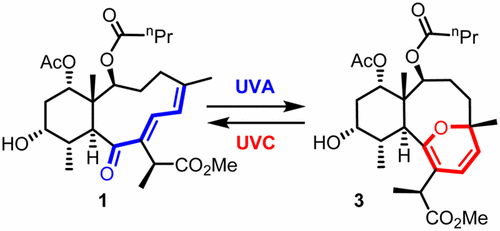
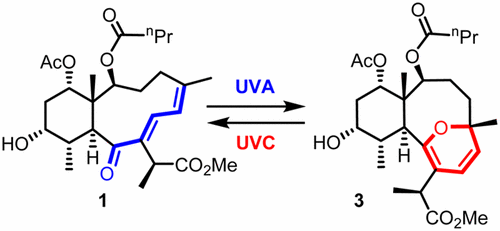
Hall, Roche, and West [OL]
Was this reversible photo-switch discovered by accidentally shining a wrong UV lamp onto the final product? The authors say it was expected. [Note: UVA = 350 nm; UVC = 254 nm]. TOC graphic:
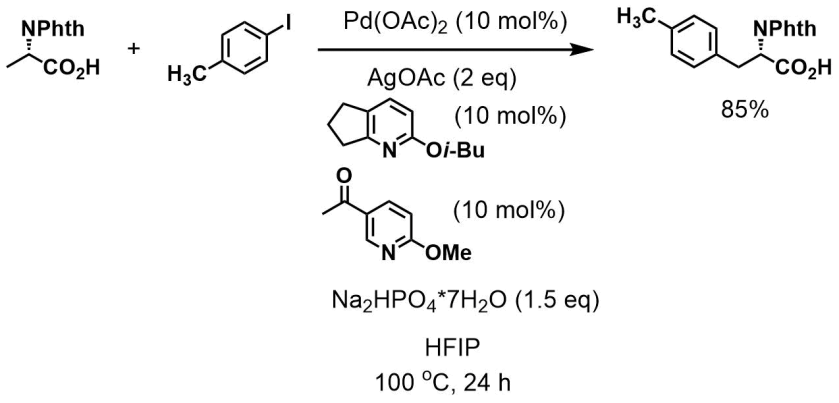
Chen et al. (Yu lab) [ACIE]
OK, enough electrocyclic reactions. Here’s some C-H activation work from Jin-Quan Yu lab. From [phthalimide-protected] alanine to [phthalimide-protected] substituted phenylalanines in one step! The conditions are somewhat peculiar though. A lot of silver (and quite a lot of palladium) was consumed for this to happen.
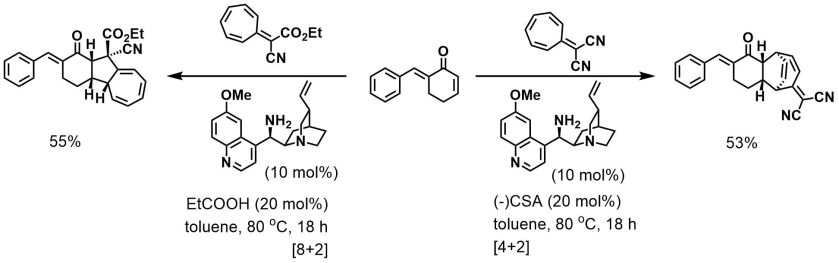
Shi, Jiang, and Tian [JOC]